Can Uloric Cause a Heart Attack?
Uloric (also known as febuxostat), a gout medication, has been a “hot topic” in headlines recently due to an announcement by the U.S. Food and Drug Administration (FDA) in February 2019 that a new black boxed warning was being added to its label. At issue is the discovery that Uloric carries a significant increase in the risk of heart attack over a commonly used alternative, Allopurinol.
At the heart of the warning is a study known as the Cardiovascular Safety of Febuxostat and Allopurinol in Participants With Gout and Cardiovascular Comorbidities (commonly referred to as the CARES Trial).
It’s common for the FDA to require drug makers to conduct ongoing aftermarket studies of their drugs. These studies help both the pharmaceutical company and the FDA understand how well, or how poorly, a drug works in real-world situations.
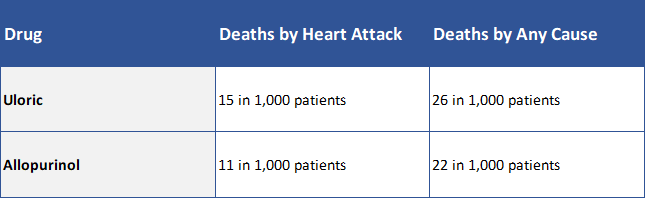
Preliminary results after seven (7) years showed that Uloric likely had an increased risk of heart-related side effects over Allopurinol. The FDA issued an alert warning doctors and patients about these initial findings in November 2017.
When the final results became available, the FDA concluded that Uloric did, in fact, have an increased risk of heart-related side effects over Allopurinol. As a result, the FDA required a black boxed warning to be added to the drug label, and the FDA advised health care professionals to only prescribe Uloric to patients for whom Allopurinol was not effective or could not be tolerated.
CARES Study Results Summary
The Rise of Uloric Lawsuits
In the few months since the FDA released its findings and required a new drug label, interest in filing Uloric lawsuits against Takeda has risen. The first case was filed in Illinois state court, but more cases are being prepared now and are expected to be filed soon.
Arguably, part of the reason we haven’t seen more lawsuits in the news is the expected move of Takeda’s U.S. headquarters from its current location in Deerfield, Illinois, to Boston, Massachusetts area. Announced in September 2018, the company is looking to consolidate closer to its large employee base in the Boston area, which has grown due to acquisitions of other health product companies over the last decade. Lack of clarity around the most appropriate venue has caused some delay in lawsuit filings, but once the move is complete, we expect to see more claims come to light.
Regardless of where lawsuits end up being filed, the company may have a hard time ahead of it. Patients who took Uloric and suffered from a heart attack, or their surviving family members, may have a strong claim against the company, given the medical evidence of the increased heart attack risk.
Filing a Uloric Lawsuit
Time to file a Uloric lawsuit is limited. If you or a loved one were injured or died while taking the gout drug Uloric, we urge you to contact us for a free case review. Our experienced Uloric attorneys and legal team will guide you through the process and answer any questions you may have regarding your case. Contact us today at 1-888-480-1123.
